CRISPR knock-outs versus knock-ins
CRISPR genome editing is accomplished using two approaches to create knock-outs via the non-homologous end joining (NHEJ) pathway or knock-ins via homology directed repair (HDR) [1]. Both approaches require two CRISPR components: the guide RNA and Cas9 nuclease. However, knock-ins require a third component—a DNA repair template. The guide RNA directs the Cas9 nuclease to insert a double strand break (DSB) within the targeted region.
Through the NHEJ pathway, the DSB ends are repaired without the need for the homologous DNA template (Figure 1). The NHEJ pathway is an error-prone mechanism resulting in frame shift mutations, which results in the disruption of a gene’s expression
and function.
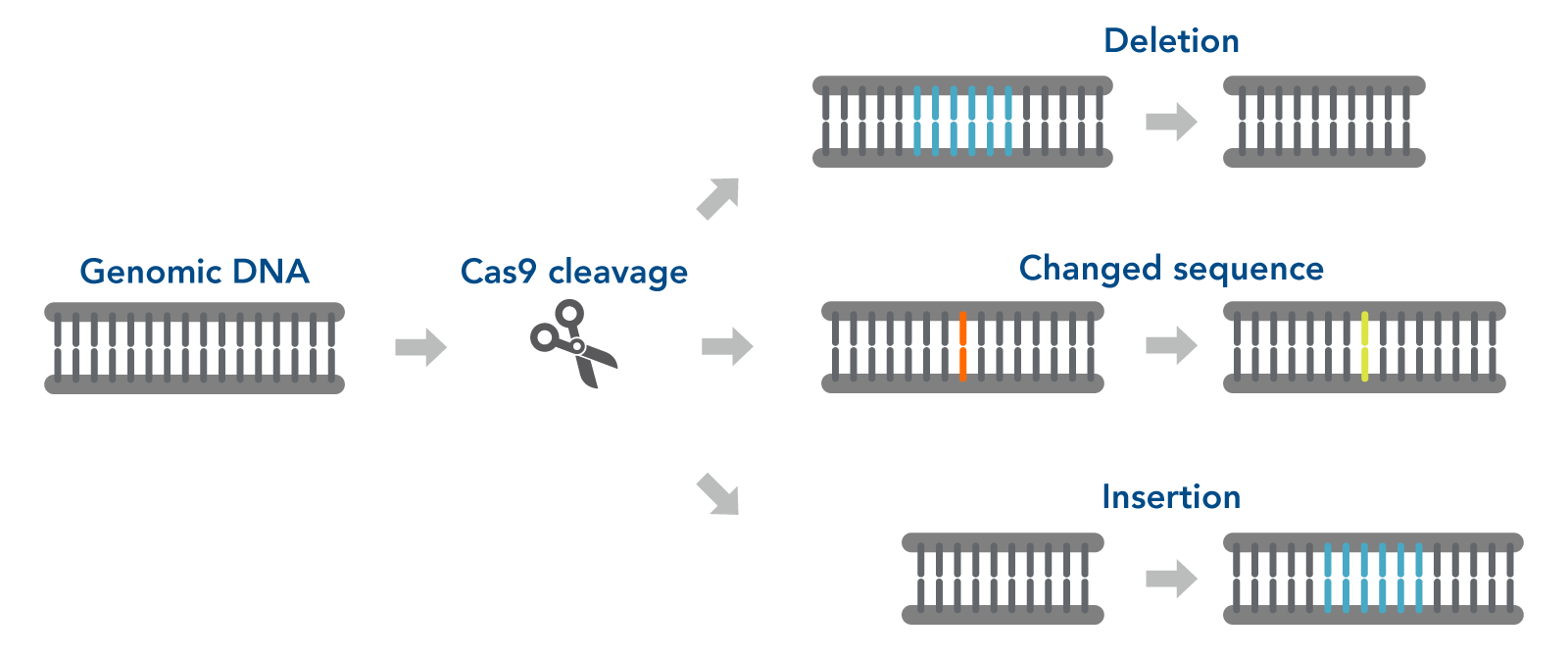
Figure 1. CRISPR-Cas9 gene editing. The guide RNA (gRNA) directs the ribonucleoprotein (RNP) to insert a double strand break (DSB) in the target DNA. The non-homologous end-joining (NHEJ) pathway joins the DSB ends without the need for a homologous template. This repair process results in the introduction of small indels at the breakpoint.
For generating gene knock-ins, if a donor repair DNA is available, the DSB can be repaired through the HDR pathway which is a non-mutagenic recombination mechanism. For more information about tips and tricks for performing knock‑in experiments, click here.
The repair of the DSB doesn’t necessarily mean perfect repair because if this occurred, it would be impossible to create gene knock-outs which is the most common application of CRISPR genome editing. To achieve clean knock-outs requires careful planning and execution, here are some key steps to maximize the efficiency and precision of your CRISPR knock‑out experiment:
- If using a two-part system, combine the crRNA and tracerRNA together to generate the guide RNA (gRNA). Heating and controlled cooling is performed to facilitate the formation of the annealed gRNA complex.
- Perform in vitro testing for efficiency to find the best one to introduce into the cells.
- To form the ribonucleoprotein (RNP) complex, combine the Cas enzyme with the complete gRNA. For Cas12a, only the crRNA is required to form the RNP complex.
- Select a method (i.e., electroporation, lipofection, microinjection) to deliver your RNP complex to your cells or model organism. The selection is dependent on various factors such as cell type, experimental requirements, and the desired efficiency.
- Electroporation involves applying brief electrical pulses to create temporary pores in the cell membrane which facilitates delivery of the RNP complex.
- Allow the cells to incubate and grow which takes about 24–48hours, but some cell types require longer incubations times, as long as 7 days.
- Analyze the samples to determine the success of your genome editing.
Discover detailed CRISPR genome editing protocols for generating gene knock-outs and knock-ins and take your research to the next level, click on the Resource tab for Genome editing with CRISPR-Cas9.
Tips for a successful knock-out experiment
Guide RNA Selection: Optimizing the Cut
gRNA selection is the foundation of a successful CRISPR knock-out experiment. Here's a detailed breakdown to help you choose the most effective guides:
- Utilize guide RNA design tools
Design tools can help select guide RNA sequences that have higher predicted on-target activity while also considering off-target editing risk. - Screen several guide RNAs
Don’t just pick one and assume it will be the most effective. Empirically test a few guide RNA designs to determine which guide has the highest editing efficiency.
- Use more than one guide RNA
You can include 2 or 3 guide RNA targeting the same region to increase the likelihood of a frameshift mutation. Frameshift mutations disrupt the reading frame of the gene leading to non-functional protein products.
- Design guide RNA that focuses on the 5’ end of the most conserved exon
Select guides that prioritize the 5’ end of the most conserved exons. This region is crucial for protein function, and edits here are more likely to create frameshift mutations that will ensure there isn’t functional protein remaining from truncated translation products or alternative spliced products.
- Utilize guide RNA design tools
Optimizing your experimental conditions
Beyond gRNA design, several experimental adjustments can significantly improve your knock-out results for better gene-editing efficiency:
- Delivery matters: Select an appropriate system such as electroporation to efficiently deliver CRISPR components (Cas9 nuclease and gRNA) into your cells.
- Concentration is crucial: Determine the optimal concentration of the gRNA-Cas9 complex for your specific cell line and experiment. Too little might result in low editing efficiency, while too much could lead to unwanted off-target editing.
- Finding the right cell density: Experiment with initial cell density. The right density can improve editing efficiency.
- Fine-tuning the gRNA: Cas9 ratio: The guide RNA to Cas9 ratio can influence editing outcomes. While a typical starting point is 1.2:1, you might need to adjust this ratio for your specific experiment.
- Delivery matters: Select an appropriate system such as electroporation to efficiently deliver CRISPR components (Cas9 nuclease and gRNA) into your cells.
By following these tips and carefully optimizing your CRISPR knock-out experiment, you can increase your chances of achieving specific gene knock-outs using CRISPR.
How IDT can help you with CRISPR experiments
IDT offers a complete CRISPR workflow from design to analysis. For more information about how to get started using CRISPR technology, download our free CRISPR Basic Handbook.


 Processing
Processing

